The Food and Drug Administration FDA has started encouraging the use of adaptive designs for clinical studies. There has been considerable interest among pharmaceutical and other medical product developers in adaptive clinical trials in which knowledge learned during the course of a trial affects ongoing conduct or analysis of the trial. Adaptive designs for medical device clinical studies.
Adaptive Designs For Medical Device Clinical Studies, What is Adaptive Design Clinical Trial. The most frequently appearing type of adaptation was the seamless Phase IIIII design 81142 57 followed by adaptive group sequential 30142 21 biomarker adaptive 28142 20 adaptive dose-finding 23142 16 pick-the-winnerdrop-the-loser 13142 9 sample size re-estimation 11142 8 adaptive randomisation 10142 7 adaptive. This final guidance applies to premarket medical device submissions including premarket approval applications PMA premarket notification. Analytical results have been derived.
 Medical Device Clinical Research Namsa From namsa.com
Medical Device Clinical Research Namsa From namsa.com
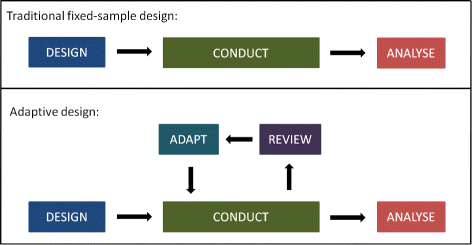
In essence adaptive designs allow prospectively planned modifications to a clinical trial based on interim data provided scientific validity the ability to draw sound inferences and data integrity credibility and reproducibility are preserved. It provides clarity on how to plan and implement adaptive designs for clinical studies used in medical device development. Stop early for effectiveness futility or safety Bayesian interim monitoring has become an accepted practice for. Adaptive design for drug and device studies.
Adaptive designs can be applied across all phases of clinical research from early-phase dose escalation to confirmatory trials.
Read another article:
Guidance for Industry and Food and Drug Administration Staff. The Food and Drug Administration FDA has started encouraging the use of adaptive designs for clinical studies. Adaptive design for drug and device studies. An adaptive design for a medical device clinical study is defined as a clinical study design that allows for prospectively planned modifications based on accumulating study data without. An adaptive design for a medical device clinical study is defined as a clinical study design that allows for prospectively planned modifications based on accumulating study data without.
 Source: researchgate.net
Source: researchgate.net
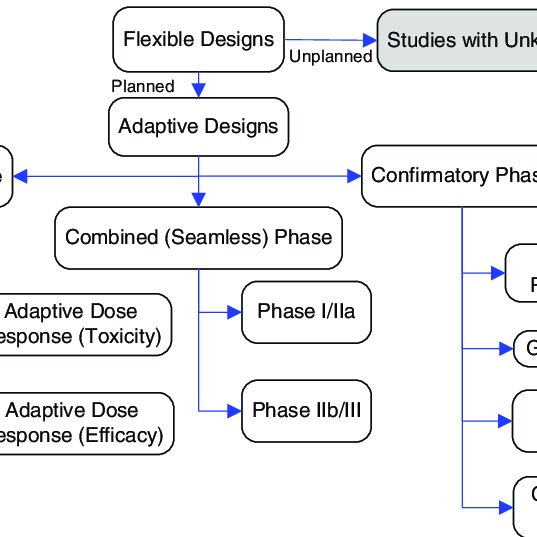
Adaptive Clinical Trial Design Case Studies. Adaptive Designs for Medical Device Clinical Studies. Barnes PJ Pocock SJ Magnussen H et al. Stop early for effectiveness futility or safety Bayesian interim monitoring has become an accepted practice for. Summary Of Different Types Of Adaptive Designs For Clinical Trials Download Scientific Diagram.
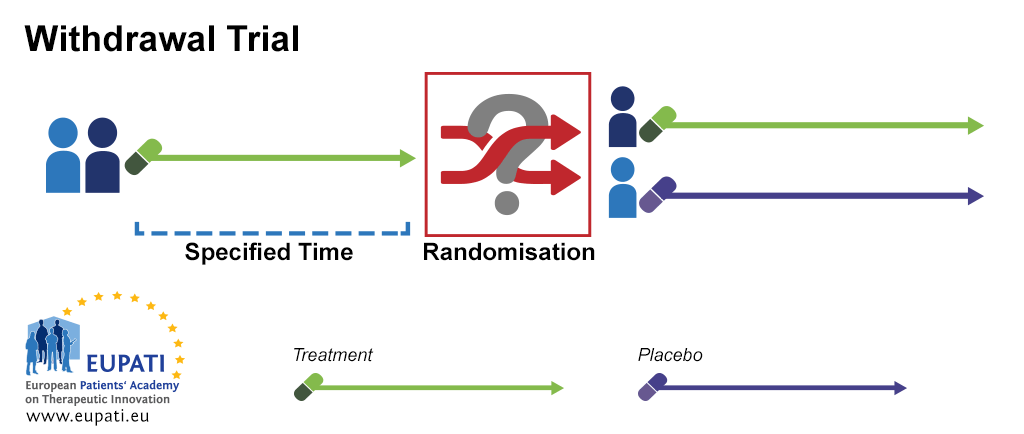 Source: toolbox.eupati.eu
Source: toolbox.eupati.eu
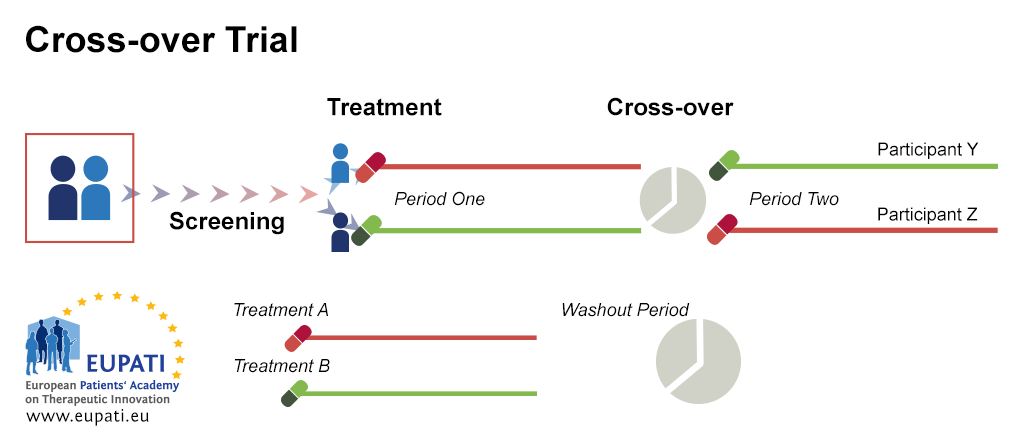
Barnes PJ Pocock SJ Magnussen H et al. An adaptive design for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications based on accumulating study data without undermining the trials integrity and validity. The EMA Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design CHMPEWP245902 defines a study design as adaptive if the statistical methodology allows the modification of a design element for example sample-size randomization ratio number of treatment arms at an interim analysis with full control of the. The 33-page guidance which finalizes a draft version released for comment in September 2018 and replaces an earlier guidance from 2010 sets out FDAs recommendations on adaptive trial design principles and the information FDA will review from adaptive studies submitted as part of investigational new drug applications INDs new drug applications. Clinical Trial Designs Eupati Toolbox.
 Source: pinterest.com
Source: pinterest.com
The US Food and Drug Administration FDA on Tuesday finalized guidance that lays out how to design medical device clinical trials that allow for changes based on data while maintaining study validity and integrity. Statistical analysis plans are needed for both interim and final analyses. An adaptive design for a medical device clinical study is defined as a clinical study design that allows for prospectively planned modifications based on accumulating study data without. In essence adaptive designs allow prospectively planned modifications to a clinical trial based on interim data provided scientific validity the ability to draw sound inferences and data integrity credibility and reproducibility are preserved. Honda Unveils Experimental Walking Assist Device With Bodyweight Support System Honda Experimental Walking Assist Devi Health Technology Medical Design Devices.
 Source: pinterest.com
Source: pinterest.com
Bringing in voices from the industrys best clinical specialists strategists and statisticians SMis 9th Adaptive Designs in Clinical Trials conference will feature case. Barnes PJ Pocock SJ Magnussen H et al. Analytical results have been derived. The EMA Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design CHMPEWP245902 defines a study design as adaptive if the statistical methodology allows the modification of a design element for example sample-size randomization ratio number of treatment arms at an interim analysis with full control of the. Pin On Services.
 Source: bmcmedicine.biomedcentral.com
Source: bmcmedicine.biomedcentral.com
An adaptive design for a medical device clinical study is defined as a clinical study design that allows for prospectively planned modifications based on accumulating study data without. In essence adaptive designs allow prospectively planned modifications to a clinical trial based on interim data provided scientific validity the ability to draw sound inferences and data integrity credibility and reproducibility are preserved. It provides clarity on how to plan and implement adaptive designs for clinical studies used in medical device development. Consequences and gains of possible trial adaptations need to be understood before initiation. Adaptive Designs In Clinical Trials Why Use Them And How To Run And Report Them Bmc Medicine Full Text.
 Source: pinterest.com
Source: pinterest.com
An adaptive design for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications based on accumulating study data without undermining the trials integrity and validity. Evaluation of medical imaging devices often involves clinical studies where multiple readers MR read images of multiple cases MC for a clinical task which are often called MRMC studies. SMi Groups Adaptive Designs in Clinical Trials Conference will return to London on 3rd and 4th April 2017 and will feature two case studies on Bayesian adaptive designs in medical device development. Adaptive design for medical devices. 10 Must Haves For Home Health Occupational Therapy Myotspot Com Orthopedics Occupational Therapy Occupational Therapist.
 Source: researchgate.net
Source: researchgate.net
The most frequently appearing type of adaptation was the seamless Phase IIIII design 81142 57 followed by adaptive group sequential 30142 21 biomarker adaptive 28142 20 adaptive dose-finding 23142 16 pick-the-winnerdrop-the-loser 13142 9 sample size re-estimation 11142 8 adaptive randomisation 10142 7 adaptive. An adaptive design for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications based on accumulating study data without undermining the trials integrity and validity. By spelling out when adaptive designs are acceptable in clinical trials for devices requiring Premarket Approval PMA or 510k premarket notification the FDA seeks to better inform both device manufacturers and its own. Key study design components can be adapted throughout the trial. Summary Of Different Types Of Adaptive Designs For Clinical Trials Download Scientific Diagram.
 Source: pinterest.com
Source: pinterest.com
There has been considerable interest among pharmaceutical and other medical product developers in adaptive clinical trials in which knowledge learned during the course of a trial affects ongoing conduct or analysis of the trial. The 33-page guidance which finalizes a draft version released for comment in September 2018 and replaces an earlier guidance from 2010 sets out FDAs recommendations on adaptive trial design principles and the information FDA will review from adaptive studies submitted as part of investigational new drug applications INDs new drug applications. Adaptive design for drug and device studies. Consequences and gains of possible trial adaptations need to be understood before initiation. Pin On Farmacevtski Proizvodi.
 Source: toolbox.eupati.eu
Source: toolbox.eupati.eu
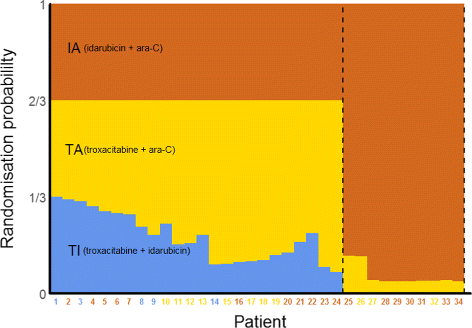
The most frequently appearing type of adaptation was the seamless Phase IIIII design 81142 57 followed by adaptive group sequential 30142 21 biomarker adaptive 28142 20 adaptive dose-finding 23142 16 pick-the-winnerdrop-the-loser 13142 9 sample size re-estimation 11142 8 adaptive randomisation 10142 7 adaptive. Reasons for Adaptive Designs in Medical Device Studies Sample size re-estimation Initial sample size based on highly uncertain effectiveness of device or control eg from feasibility or historical studies. The pharmaceutical landscape is evolving as stakeholders pursue the use of real-world evidence precision medicine and complex innovative trial designs CID to increase efficiency of development. This final guidance applies to premarket medical device submissions including premarket approval applications PMA premarket notification. Clinical Trial Designs Eupati Toolbox.
 Source: jliedu.com
Source: jliedu.com
An adaptive design for a medical device clinical study is defined as a clinical trial design that allows for prospectively planned modifications based on accumulating study data without undermining the trials integrity and validity. The pace of the uptake of adaptive designs in clinical research however has remained well behind that of the statistical literature introducing new methods and highlighting their potential advantages. The purpose is to. Analytical results have been derived. Adaptive Design Clinical Trials Jli Blog.
 Source: pinterest.com
Source: pinterest.com
Adaptive design for medical devices. The US Food and Drug Administration FDA on Tuesday finalized guidance that lays out how to design medical device clinical trials that allow for changes based on data while maintaining study validity and integrity. SMi Groups Adaptive Designs in Clinical Trials Conference will return to London on 3rd and 4th April 2017 and will feature two case studies on Bayesian adaptive designs in medical device development. By spelling out when adaptive designs are acceptable in clinical trials for devices requiring Premarket Approval PMA or 510k premarket notification the FDA seeks to better inform both device manufacturers and its own. Pin Na Doske Interface Design.
 Source: cancertreatmentreviews.com
Source: cancertreatmentreviews.com
This final guidance applies to premarket medical device submissions including premarket approval applications PMA premarket notification. It provides clarity on how to plan and implement adaptive designs for clinical studies used in medical device development. We develop adaptive MRMC design methodologies to enable study resizing. An adaptive design for a medical device clinical study is defined as a clinical study design that allows for prospectively planned modifications based on accumulating study data without. New Clinical Trial Designs In The Era Of Precision Medicine An Overview Of Definitions Strengths Weaknesses And Current Use In Oncology Cancer Treatment Reviews.
 Source: bmcmedicine.biomedcentral.com
Source: bmcmedicine.biomedcentral.com
It provides clarity on how to plan and implement adaptive designs for clinical studies used in medical device development. Adaptive Clinical Trial Design Case Studies. Guidance for Industry and Food and Drug Administration Staff. Adaptive Designs for Medical Device Clinical Studies. Adaptive Designs In Clinical Trials Why Use Them And How To Run And Report Them Bmc Medicine Full Text.
 Source: veristat.com
Source: veristat.com
An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. An adaptive design is defined as a design that allows modifications to the trial andor statistical procedures of the trial after its initiation without undermining its validity and integrity. Barnes PJ Pocock SJ Magnussen H et al. By spelling out when adaptive designs are acceptable in clinical trials for devices requiring Premarket Approval PMA or 510k premarket notification the FDA seeks to better inform both device manufacturers and its own. What To Know Before Considering An Adaptive Design Clinical Trial.
 Source: pinterest.com
Source: pinterest.com
The US Food and Drug Administration FDA on Tuesday finalized guidance that lays out how to design medical device clinical trials that allow for changes based on data while maintaining study validity and integrity. Adaptive design for medical devices. Adaptive designs can be applied across all phases of clinical research from early-phase dose escalation to confirmatory trials. In essence adaptive designs allow prospectively planned modifications to a clinical trial based on interim data provided scientific validity the ability to draw sound inferences and data integrity credibility and reproducibility are preserved. Blink Home Medical Device In 2021 Medical Device Design Devices Design Design Awards.